A group of researchers has unearthed the secrets behind a tiny but crucial protein that shuttles zinc ions (Zn2+) within our bodies. The discovery offers a deeper understanding of how our cells maintain optimal health.
Zn2+ may be small, but they play a mighty role in our cells. Zinc enables enzyme catalysis, protein folding, DNA binding, and regulating gene expression, with about 10% of the proteins in our body reliant on Zn2+ to function effectively.
The study, which was published in the journal Nature Communication on August 8, 2023, focused on the Golgi apparatus – a cellular compartment that processes, sorts and distributes cells to their final destination. Within the Golgi, three distinct zinc transporter (ZnT) complexes – ZnT4, ZnT5/6, and ZnT7 – collaborate to usher Zn2+ ions from the cellular interior (cytosol) into the Golgi. While these complexes have long been known to play pivotal roles, the precise mechanisms governing Zn2+ transport within them have remained an enigma.
“We concentrated our study on the transport protein hZnT7,” says Kenji Inaba, a corresponding author of the study and professor at Tohoku University’s Institute of Multidisciplinary Research for Advanced Materials Sciences. “The study built upon our previous research that hZnT7 plays a vital role in Zn2+ uptake into the cis-Golgi cisterna and regulates the localization, traffic and function of the chaperone protein ERp44.”
Cryo-EM map of human ZnT7 in complex with Fab at 2.2 a resolution. ©Han Ba Bui and Kenji Inaba
To reveal more about hZnT7, Inaba and his colleagues employed an advanced technique called cryo-electron microscopy (cryo-EM). Two cryo-EM machines, one from Tohoku University and one from the University of Tokyo, could capture detailed images of hZnT7 in action. By using a Fab fragment from a monoclonal antibody that specifically binds hZnT7 the researchers succeeded in determining the cryo-EM structures of hZnT7 at near-atomic resolutions, gaining critical insights into the mechanisms of Zn2+ transport.
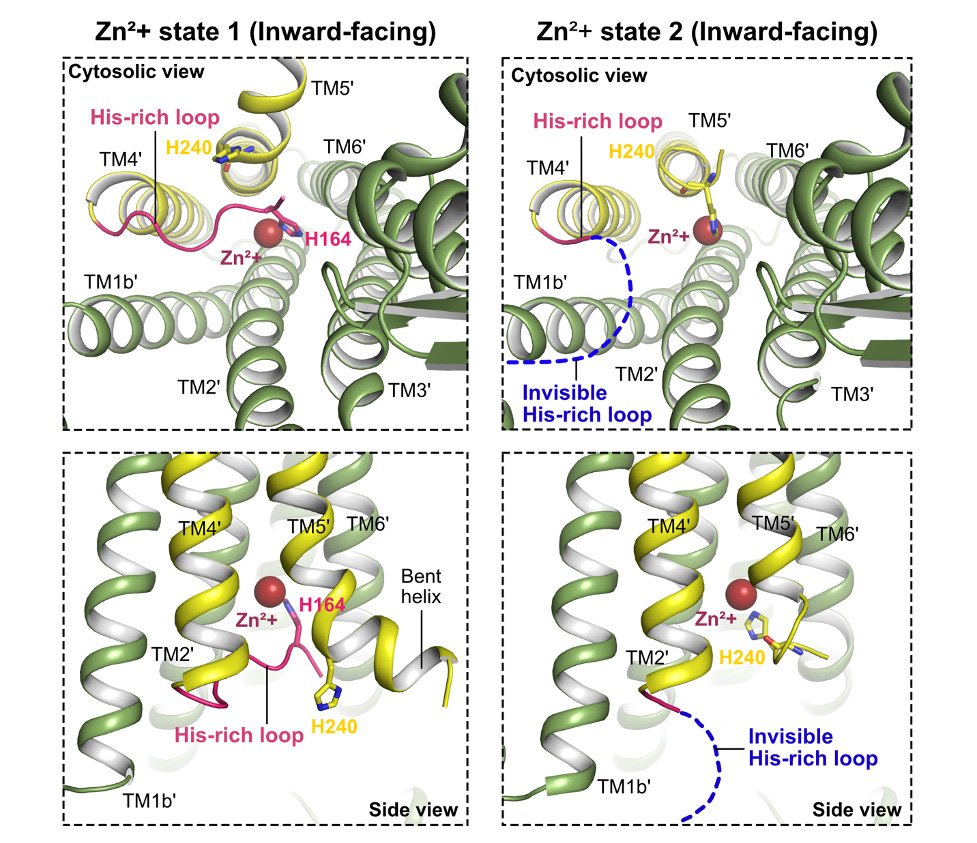
Comparative analysis between hZnT7 and other zinc transporters, including human ZnT8 and bacterial YiiP, exposed distinct structural features of hZnT7. The existence of hZnT7 as a homodimer with varying Zn2+ -bound configurations holds particular significance. Notably, hZnT7 boasts an elongated cytosolic histidine-rich loop (His-loop) that interfaces with the transmembrane metal-binding site, a vital feature governing zinc transfer. During Zn2+ recruitment via the His-loop, hZnT7 undergoes intricate conformational rearrangements, shedding light on an unparalleled mechanism of zinc transport.
Conformational transition of the hZnT7 protomer during the Zn2+ transport from the cytosol to the Golgi lumen. ©Han Ba Bui and Kenji Inaba
It is widely known that hZnT7 is a key player in dietary zinc absorption and controlling body fat. When zinc levels drop in certain parts of the body, it can lead to issues like prostate cancer development in mice and disruptions in how our bodies process insulin.
Inaba adds their findings will result in greater understandings of the molecular processes with certain pathogens. “With it reported that abnormalities in Golgi-resident ZnT transporters result in fatal diseases such as diabetes, cancers, and immunodeficiency, it’s essential to understand the pathogenic mechanisms of these diseases at the molecular and cellular level.”
The histidine-rich loop segment is inserted into the cytosolic cavity and coordinated to Zn2+ at the transmembrane metal-binding site for efficient Zn2+ uptake. ©Han Ba Bui and Kenji Inaba
Publication Details
“Cryo-EM structures of human zinc transporter ZnT7 reveal the mechanism of Zn2+ uptake into the Golgi apparatus”
Han Ba Bui, Satoshi Watanabe, Norimichi Nomura, Kehong Liu, Tomoko Uemura, Michio Inoue, Akihisa Tsutsumi, Hiroyuki Fujita, Kengo Kinoshita, Yukinari Kato, So Iwata, Masahide Kikkawa & Kenji Inaba
Nature Communications
Date of Publication: August 8th, 2023
DOI: 10.1038/s41467-023-40521-5
▶ Pressrelease in Japanese
▶ Tohoku University
▶ Biomolecular Structure (INABA LABORATORY)
Contact
Institute of Multidisciplinary Research for Advanced Materials
Email: kenji.inaba.a1[at]tohoku.ac.jp
Website: https://www2.tagen.tohoku.ac.jp/lab/inaba/html/